


Donate Now
and become
Forum Supporter.

Many perks!
<...more...>


|

05-04-2009, 12:11 PM
|
|
Member
|
|
Join Date: May 2009
Location: St Petersburg, FL
Posts: 45
|
|
 Flasking Frustrations, Finally Feeling Fed up
Flasking Frustrations, Finally Feeling Fed up
Okay, so I'm not actually fed up, I just wanted to use all that alliteration.  But I AM frustrated. I've made 2 attempts at home-flasking in a sterile glovebox, and got bad results. I don't actually think that my problem is contamination, however. I understand sterile procedure, and found several step-by-step flasking procedures online and used them to design my own sterile flasking checklist and procedure. It seemed pretty solid, but I'm left with a bunch of jars full of useless media.
Typing out everything that I did and where I think I went wrong would be WAY too long for a thread, so I guess I'll need someone to email back-and-forth with me a little. My addy is bigsubjo@msn.com, just send me an email with something orchidy in the subject and I'll hit you back with the rundown of my procedure/results. I'd really prefer a response from people who have experience successfully flasking at home, if y'all don't mind. Thanks in advance, I can't wait to bring bunches and bunches of little orchids into the world!

|

05-04-2009, 12:21 PM
|
|
Senior Member
|
|
Join Date: Jan 2008
Zone: 8a
Location: Piney Woods of East Texas
Age: 47
Posts: 3,253
|
|
Sorry, I double posted. See next post.
Last edited by Royal; 05-04-2009 at 12:24 PM..
Reason: Double Post - oops.
|

05-04-2009, 12:22 PM
|
|
Senior Member
|
|
Join Date: Jan 2008
Zone: 8a
Location: Piney Woods of East Texas
Age: 47
Posts: 3,253
|
|
I understand your frustrations, but I didn't catch your problem. Could you clarify a few things for us?
First, what type of seed are you sowing? Why do you believe that contamination is not the issue? What do you mean by "useless media?" How long have the seeds been on the medium?
You don't have to write a dissertation, just fill in some blanks. There are a few of us here that flask at home, I'm sure we can help pin down your problems. We just need some info.
|

05-04-2009, 05:49 PM
|
|
Member
|
|
Join Date: May 2009
Location: St Petersburg, FL
Posts: 45
|
|

-I'm mostly going to be growing cattleya-alliance, the ones I tried to sow were GREEN PODS of E. tampensis (don't know if green pod would make a difference over dry seed. I waited til some pods were dehiscing, and then took the green ones that were left). The media I purchased from Phytotech Labs was listed as being for Catts and their kin.
-I don't believe that contamination is the issue because I don't see any bacterial or fungal growth- nothing fuzzy or patchy on the surface, just liquid and discoloration which I'll explain in just a sec. The jar I later opened had no odor to it- nothing sour or musty or moldy. Just the clean banana-y smell of the medium.
-I mean "useless" because it hasn't grown any plants, and I sure can't try to use it again.
-The seeds were under fluorescent lights for months (at least 2, probably 3) on a 13-hour-a-day light cycle. Some research suggested 12 hours, other places suggested 14. I split the difference.
The discoloration seemed to be from condensation. In the 1st batch, water that had been clinging to the jar's sides rolled down onto the medium in each jar, and where the water sat, the gel turned whitish gray, as opposed to the almost black it had been. Within days, the whole surface had gone runny with moisture and subsequently discolored. Again, no fuzz, no patches, no smell, no brown or yellow, just the wet grayness.
For the second batch, I used some sterile gauze pads to wipe the inside of the jar and blot the medium surface to get rid of excess moisture. But still, within days I had water pooling, and then discoloration under the water, and no protocorm growth even for months after.
IF it is contamination, it is occuring at the point when I mix the dry powder with the boiling water, but I immediately put the media-filled jars into a steam bath for 30 min after they have been filled. The jars are then left unopened until they are in the sterile box and have been wiped down with 10% bleach/water (along with everything else).
Is condensation my issue? Am I mixing my medium with too much water, despite following the directions exactly? I want to cry a little, I think.
If my next attempts don't work out, it will be more cost-effective for me to just send my pods out and have them sown professionally. But I'm a DIY kind of guy, and would feel very bitter about having to throw in the towel.

|

05-04-2009, 06:04 PM
|
 |
Administrator
|
|
Join Date: Oct 2007
Location: middle of the Netherlands
Posts: 13,779
|
|
I don't have any experience with flasking (but will be doing so very soon!) so don't know about condensation being an issue, but have you considered the viability of your seeds?
__________________
Camille
Completely orchid obsessed and loving every minute of it....
My Orchid Photos
|

05-04-2009, 06:13 PM
|
|
Senior Member
|
|
Join Date: Feb 2009
Zone: 9a
Location: Orlando
Posts: 210
|
|
I started flasking recently too. I think the key is to try various seeds because some simply are not viable. So far out of 9 flask and 8 different seed types, I have 2 flasks that have germinated. The odds are in your favor if you try other seeds. You can get seeds very cheaply at the Orchid Seed Bank project as well as inexpensive media. It sounds like you are pretty sure your technique was good so maybe the seeds simply were not? If you go to the seed bank try their Cattleya grossi. I just germinated some from them.
Pat
|

05-05-2009, 09:56 AM
|
|
Senior Member
|
|
Join Date: Jun 2007
Location: Virginia
Posts: 153
|
|
JonnyBrabo,
1. Contamination: I don't think so: " no fuzz, no patches, no smell, no brown or yellow"
2. Condensation: I hope not. My friends and I got the same problem with condensation but our seeds germinate well (or better with condensation). If you don't like condensation then wait for few days for water to lay on the top of media and take it out using a pipette.
3. Seed viable & mature: Are you sure that your seeds are viable & mature enough? How long is your pod? Normal Cat. seeds may take a year to be ripen (if I remember correctly). Try mature seed.
4. Check pH: pH around 5.1 is the best. 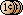 
Last edited by newflasker; 05-05-2009 at 10:01 AM..
|

05-05-2009, 10:16 AM
|
|
Senior Member
|
|
Join Date: Jan 2008
Zone: 8a
Location: Piney Woods of East Texas
Age: 47
Posts: 3,253
|
|

Ok Johnny, don't throw in the towel. It sounds like you've done your homework. You can totally do this, you may just need to tweak a few things in your process.
First, Pat makes a good point. If the seed is not viable, nothing else matters. I like your logic on harvesting times of the green capsules - I bet they are ok, but one can never know for sure without microscopic examination.
As far as procedure goes, I see one fatal flaw that leads me to believe that contamination is the problem - the steam bath. Boiling water is not hot enough to effectively sterilize. Once water hits 212 it boils, loosing energy in the form of steam and can not get any hotter. This sanitizes, but doesn't sterilize.
This method is OK for canning some acidic foods in a brine (that further preserves the food). But to preserve meats, fruit, or make flasks, higher temps have to be achieved to kill heat resistant spores and cysts. Contamination comes in all forms. Bacterial contam looks very different from fungal, as fungal looks different from algal. Something is liquefying your media. Water won't do that, it will just float on top.
You MUST use a pressure cooker or autoclave. Cook at 15 psi for at least 15 minutes and everything dies.  Exerting pressure raises the boiling temperature of water so it climbs to ~230 before evaporating.
Try this out and let the flasks sit for a while to make sure they're contam-free before opening them. The media shouldn't be a problem for E. tampensis. I'd be a little curious to see the same batch of media prepared in a p-cooker. But if you must use different media, just order from OSP. One liter packets are cheap and no exorbitant shipping like Phytotech. Plus while you're there you can purchase a $4 seed pack of some seed with previous reports of good germination.
Good luck, and keep us posted. Keep at it. You'll have jars full in no time. 
Last edited by Royal; 05-05-2009 at 10:22 AM..
|

05-05-2009, 11:31 AM
|
|
Senior Member
|
|
Join Date: Sep 2008
Location: Queensland
Age: 54
Posts: 623
|
|
Hi
On the note of if the seeds are good or not, it is possible to produce masses of what looks like great seed and yet it is a dud. Some years ago I got a pod with Euchile Citrina x Epidendrum radicans. to work. It grew to maturity and started to split. I sent it to a friend to flask. He called to say, nice looking pod but the seeds had no embryo inside. Basically, a husk and no viable stuff inside to grow.
I lately have put in several intergeneric crosses between distantly related species and complex hybrids. The lab has yet to report growth on any, but did say there was not a lot of viable seed in the pods.
As far as I know, even the experts have stuff not germinate. Just keep trying. It sounds like you are doing the right things.
Brett
|

05-05-2009, 11:36 AM
|
|
Member
|
|
Join Date: May 2009
Location: St Petersburg, FL
Posts: 45
|
|
Thanks for all your suggestions, folks! I'm feeling bolstered now, and well-armed with all the ideas you've passed on. Now I've just got to wait for my pods to ripen so I can try again- think I'll see if the dentist's office where my mom works will 'clave my jars for me. You guys rock! I just joined this site the other day, and I've already found nearly endless information and resources!
|
|
Currently Active Users Viewing This Thread: 1 (0 members and 1 guests)
|
|
|
 Posting Rules
Posting Rules
|
You may not post new threads
You may not post replies
You may not post attachments
You may not edit your posts
HTML code is Off
|
|
|
All times are GMT -4. The time now is 06:46 PM.
|



















 But I AM frustrated. I've made 2 attempts at home-flasking in a sterile glovebox, and got bad results. I don't actually think that my problem is contamination, however. I understand sterile procedure, and found several step-by-step flasking procedures online and used them to design my own sterile flasking checklist and procedure. It seemed pretty solid, but I'm left with a bunch of jars full of useless media.
But I AM frustrated. I've made 2 attempts at home-flasking in a sterile glovebox, and got bad results. I don't actually think that my problem is contamination, however. I understand sterile procedure, and found several step-by-step flasking procedures online and used them to design my own sterile flasking checklist and procedure. It seemed pretty solid, but I'm left with a bunch of jars full of useless media.






 Exerting pressure raises the boiling temperature of water so it climbs to ~230 before evaporating.
Exerting pressure raises the boiling temperature of water so it climbs to ~230 before evaporating.






 Linear Mode
Linear Mode


