


Donate Now
and become
Forum Supporter.

Many perks!
<...more...>


|

01-10-2022, 01:59 PM
|
 |
Senior Member
|
|
Join Date: May 2005
Location: Oak Island NC
Posts: 15,302
|
|
 Root Death
Root Death
This is a new thread, started at Keith's good recommendation, as separate from the one on probiotics.
Quote:
Originally Posted by K-Sci

But Ray, how much oxygen do you think there is in stagnant water in an gas-impermeable glass container? My inclination is to conclude that the success of S/H and H proves that suffocation is not the mechanism that causes root death in broken down media. This was the genesis of my post months ago speculating on a variety of other possible factors that could cause orchid root death in old media. I'm not saying you're wrong, but only that it appears to me that the evidence suggests that you are wrong.
Is it possible to measure the O2 in a beaker without changing the result when getting a sample?
-Keith
|
Below is my chain of thought that led me to the "suffocation" suspicion.
Many years ago, when I was developing the S/H technique for orchids, Rod Venger (Venger's Orchids in Colorado Springs) began playing with what is now called "water culture". He had some contacts with (I think) Texas A&M and a PhD candidate there pointed out that orchids roots modify their cell structure to function optimally in the environment into which they are growing, and that, once grown, they cannot change, which explains quite well the need for a plant to basically grow a whole new root system when there is a big change in the medium conditions.
As part of both of our experiments, we noted that existing roots from "dry" media, when submerged in water, would die. That seemed to follow from the "they cannot change" concept, and our best guess was "drowning", AKA "suffocation". That was further supported when we noted that roots that grew into the water on their own were perfectly fine and did not drown.
It is also well-established that if too fine of a potting medium is used and the pot is overwatered, the roots can fail, even if the potting medium if fresh and not decomposing.
All of those scenarios combine to point to suffocation, in my mind.
Consider the case in which a plant is moved from one potting medium into another - for example, from a bark-based medium into sphagnum, as an attempt to not have to water so often. The sphagnum, likely being more moisture-retentive, becomes an inhospitable, possibly suffocating, environment for the existing roots, but not so extreme to cause rapid failure, giving the roots time to grow viable "optimized" roots within the moss. (See the attached photo.)
However, there is another scenario that may-, or may not absolutely point to suffocation, but certainly supports that possibility, if you so choose: Looking at that same image, what if that were a case in which the roots grew well in fresh medium. Over time, however, the medium began to decompose, becoming finer and finer, suffocating those roots. But wait! The new root growth, with the upper parts having been given a "stay of execution" by the slowly decomposing medium, were able to successfully grow in it.
It seems to me that the second scenario is a lot like roots growing into the reservoir in a S/H pot, and the two scenarios combined suggests that it's not a pathogen or "toxic chemistry" situation that's the issue.
Might it be something other than suffocation? Sure, and if that's the case, I'd love to understand the mechanism, but it sure looks that way to me.
Last edited by Ray; 01-10-2022 at 02:03 PM..
|
|
Post Thanks / Like - 3 Likes
|
|
|
|
|

01-10-2022, 05:23 PM
|
 |
Senior Member
|
|
Join Date: Sep 2020
Zone: 8a
Location: Central Mississippi
Posts: 653
|
|

Quote:
Originally Posted by Ray

This is a new thread, started at Keith's good recommendation, as separate from the one on probiotics.
Below is my chain of thought that led me to the "suffocation" suspicion.
Many years ago, when I was developing the S/H technique for orchids, Rod Venger (Venger's Orchids in Colorado Springs) began playing with what is now called "water culture". He had some contacts with (I think) Texas A&M and a PhD candidate there pointed out that orchids roots modify their cell structure to function optimally in the environment into which they are growing, and that, once grown, they cannot change, which explains quite well the need for a plant to basically grow a whole new root system when there is a big change in the medium conditions.
As part of both of our experiments, we noted that existing roots from "dry" media, when submerged in water, would die. That seemed to follow from the "they cannot change" concept, and our best guess was "drowning", AKA "suffocation". That was further supported when we noted that roots that grew into the water on their own were perfectly fine and did not drown.
It is also well-established that if too fine of a potting medium is used and the pot is overwatered, the roots can fail, even if the potting medium if fresh and not decomposing.
All of those scenarios combine to point to suffocation, in my mind.
|
Perhaps we can work together to reason through some of this to gain more insight -- my fear is that it will just raise more questions. 
There are some major principles I can think of apply here.
1) A large volume filled with small spheres and water contains the same percent water regardless of the sphere size. This principle doesn't hold true for a volume filled with spheres of various sizes because settling is possible The small spheres can fill in the gaps between the larger
Extending this to potting media, the implications for LECA spheres are pretty obvious. To some extent, the same principles should apply at least to some extent to straight bark chunks assuming it has not compacted to fit the pieces more closely.
2) Next, in such a volume, as fill ratio (density) increases the area though which O2 can diffuse through the water goes down. The logical conclusion is that size-graded chunk media should be more open than orchid mixes (this is pretty easy to see). Potting mixes and potting soil have widely varied particle sizes, so we can expect far less air diffusion area to be available, which would tend to interfere with, oxygen, C02, or microbe waste product diffusion.
But there are other effects as described here: http://compost.css.cornell.edu/oxygen /oxygen.diff.water.html one is that "When oxygen is forced to diffuse through water saturated pores, this restriction on oxygen transport quickly leads to anaerobic conditions." We know that a volume of small particles has more surface area on which oxygen-consuming microbes could grow compared to large particles. Both the volume of the microbes and their use of oxygen would impact oxygen availability to roots when the particle size is small. This would obviously lead to root suffocation.
3) Would the breakdown of large particles into smaller ones by the microbes. Yet another factor could be pH and chemistry changes to the water the gaps.
4) A mechanism that doesn't require O2 suffocation - would there be toxins produced by anaerobic microbes whereby it is not the lack of O2 but the microbe wastes that cause root death?
Quote:
|
Consider the case in which a plant is moved from one potting medium into another - for example, from a bark-based medium into sphagnum, as an attempt to not have to water so often. The sphagnum, likely being more moisture-retentive, becomes an inhospitable, possibly suffocating, environment for the existing roots, but not so extreme to cause rapid failure, giving the roots time to grow viable "optimized" roots within the moss. (See the attached photo.)
|
I've read your sticky on roots, and think the observations and reasoning are sound. That's a very interesting and worthwhile manuscript.
...but since you bring up sphagnum I'll offer some thought on this. I think may works against my original doubt that straight up suffocation is what usually kills the roots.
I have wondered why sphagnum doesn't immediately result in root suffocation the first time it is over-watered. Instead, roots in fresh sphagnum no mater how compressed, seem to be very resistant to over-watering death. Why?
Sphagnum has antiseptic qualities that could prevent the growth of aerobic microbes and thereby prevent or delay root suffocation. Sphagnum also has vast numbers of internal channels that transport water internally, which explains why it will wick up water and distribute it more or less uniformly inside. If the sphagnum is not saturated, these channels are available to transport air far more freely than diffusion through water.
Quote:
|
However, there is another scenario that may-, or may not absolutely point to suffocation, but certainly supports that possibility, if you so choose: Looking at that same image, what if that were a case in which the roots grew well in fresh medium. Over time, however, the medium began to decompose, becoming finer and finer, suffocating those roots. But wait! The new root growth, with the upper parts having been given a "stay of execution" by the slowly decomposing medium, were able to successfully grow in it.
|
Wow, I love this observation!. It isn't something I've considered before, but you're definitely right about the root changes. I'll have to mull this one over. Off the cuff, couldn't the smaller roots be the result of smaller spaces, a build up of toxins, depletion of nutrients by microbes, or just the different root types have different sizes? The new roots look more like aerial roots in the gaps that are more open.
Quote:
It seems to me that the second scenario is a lot like roots growing into the reservoir in a S/H pot, and the two scenarios combined suggests that it's not a pathogen or "toxic chemistry" situation that's the issue.
|
During this discussion it occurred to me that there is a bit of a semantic problem. If microbes use all the oxygen in the mix, is the root death caused by suffocation, or by the microbes? Really it is both.
Likewise, if C02 build up in media that can't breath results in acidification death of the roots, is it suffocation or chemistry? We may agree on more than we think.
Quote:
Might it be something other than suffocation? Sure, and if that's the case, I'd love to understand the mechanism, but it sure looks that way to me.
|
See my comments above.
-Keith
__________________
+++++++++++
Last edited by K-Sci; 01-10-2022 at 05:26 PM..
|

01-10-2022, 08:11 PM
|
 |
Senior Member
|
|
Join Date: May 2005
Location: Oak Island NC
Posts: 15,302
|
|

I haven't read the whole post yet, but this caught my eye:
Quote:
A large volume filled with small spheres and water contains the same percent water regardless of the sphere size. This principle doesn't hold true for a volume filled with spheres of various sizes because settling is possible The small spheres can fill in the gaps between the larger
Extending this to potting media, the implications for LECA spheres are pretty obvious. To some extent, the same principles should apply at least to some extent to straight bark chunks assuming it has not compacted to fit the pieces more closely.
|
That is, unfortunately, untrue. Yes, the percentage of the volume filled by the spheres, and that remaining unfilled is the same in total, but as the pore size decreases, the percentage filled by "bridging water", held by surface tension, increases.
LECA is a pretty good "sponge", so sucks away that water and spreads it around, but there does reach a point where the pellets are saturated, and the bridging water becomes an issue.
With other media ingredients, the void spaces take a wide variety of different shapes, but the same principle applies, and if the uniform, non-spherical particles ultimately become "stacks of cards" rather than "card houses", there will be more bridging water, and that will certainly be the case when you mix sizes and shapes.
Particle packing was in the most basic ceramic processing courses, and even though that was a long time ago...
---------- Post added at 07:11 PM ---------- Previous post was at 05:26 PM ----------
On to point 2 - if I recall, oxygen diffuses at about 1-2 mm/sec, so I cannot see that as being an issue unless the pathway from the surface down to the roots is extended by broken-down media - but that would be suffocation. I suspect air is the primary fluid through which the roots interact with gases - in an out - so restriction would lead to suffocation or poisoning.
Next - do we have any idea of the population density of the microbes in the medium, and do we have any grasp on the volume of oxygen they might demand?
Personally, I think this might be a lot of mental masturbation that leads back to something close to suffocation, if not exactly that, and really may not add much to the decisions we make in out culture.
Interesting? Always! Valuable? I'm not so sure.
Next... |

01-10-2022, 11:52 PM
|
|
Member
|
|
Join Date: Dec 2021
Posts: 94
|
|
Why do the roots need oxygen? They can carry out anaerobic respiration and I don't think they have high energy jobs to do to maintain life (there are plenty of anaerobic algae. It all started that way so anaerobic function is the IBM-DOS buried in the computer program).
I don't know what the answer. Is it resistance to external toxins, buildup of metabolic toxins, failure to carry out some crucial oxygen dependent biochemical process?
|

01-11-2022, 01:00 AM
|
 |
Senior Member
|
|
Join Date: Mar 2011
Location: Albuquerque New Mexico
Posts: 1,014
|
|

I'm not basing this on any real science as you guys seem to be but my instinct is that the roots do not die from a microbial problem but the change in conditions directy contributes to the roots death, perhaps in different ways.
If the root has developed under water, perhaps removing it either leads to dessication, or perhaps even by being exposed to a poisonous level of oxygen in the air. Or perhaps the structure of the velamin adapted to being underwater is just not suited to ever dry out. If velamin adapts to shield the root from one condition, it may be unable to shield it from the perils of another condition.
None of these things are suffocation but they are also no related to microbial activity.
I also feel that people tend to assume that water in S/H culture is stagnant. In my mind the constant flushing and replacement of water keeps the whole pot light and fresh. I tend to assume that there are far less anaerobic bacteria in S/H than most other styles of potting.
Sorry if that was rant and I hope it made some sense. All that said stagnant water and bacteria also must kill roots of course but I have a sense they are not usually the cause of root death in repottings and situation changes

|

01-11-2022, 01:28 AM
|
|
Senior Member
|
|
Join Date: Dec 2018
Location: Australia, North Queensland
Posts: 5,212
|
|
I haven't tried it yet. But wondering if an aquarium air-stone pump system for water surface air mixing can help with keeping regular roots alive - by keeping the water adequately oxygenated. But ..... my main reason for not going for water culture (not 'S.H.') is the need for fixing the position of the orchid in its water container. Maybe a bit fiddly if too many orchids.
|

01-11-2022, 08:56 AM
|
 |
Senior Member
|
|
Join Date: May 2005
Location: Oak Island NC
Posts: 15,302
|
|
When I first was thinking about the “suffocation” of roots in media that was too fine - whether that be due to a case of poor medium choice or it becoming old and compressed, the term I used was “stifling gas exchange”, which is pretty nonspecific in regards to what gases and in what direction.
I suppose that over the years that has been shortened to “suffocation”.
|

01-11-2022, 12:56 PM
|
 |
Senior Member
|
|
Join Date: May 2005
Location: Oak Island NC
Posts: 15,302
|
|
I want to go back and clarify a response I made earlier about spheres and particle packing.
Keith was absolutely correct that the size of spheres makes no difference to the relative filled and open volumes - fill a room with basketballs or ping-pong balls, and it's the same.
What I misinterpreted was his statement that the void space is filled with water... I was not thinking of his theoretical example, but of a potted plant, where the void is not filled with water, but air, with some water held in the tiny spaces between particles.
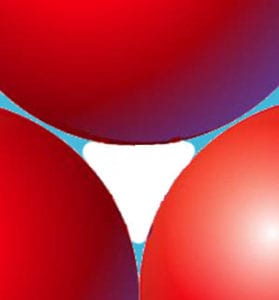
In that context, it seems obvious that the smaller the particles, the smaller the voids, so more of the open volume is occupied by water, leading to stifled gas exchange/suffocation (choose one). |

01-11-2022, 03:45 PM
|
 |
Senior Member
|
|
Join Date: Sep 2020
Zone: 8a
Location: Central Mississippi
Posts: 653
|
|

Quote:
Originally Posted by Louis_W

I'm not basing this on any real science as you guys seem to be but my instinct is that the roots do not die from a microbial problem but the change in conditions directy contributes to the roots death, perhaps in different ways.
|
I definitely agree with both you and Ray on this point. It seems that changing potting media has a much larger impact on roots than would seem to be attributable to injury. My experience with S/H is putting a couple seedlings in it as an experiment so I cannot comment on this aspect.
Quote:
If the root has developed under water, perhaps removing it either leads to dessication, or perhaps even by being exposed to a poisonous level of oxygen in the air. Or perhaps the structure of the velamin adapted to being underwater is just not suited to ever dry out. If velamin adapts to shield the root from one condition, it may be unable to shield it from the perils of another condition.
|
If you haven't, you may want to read Ray's sticky post on roots.
Quote:
None of these things are suffocation but they are also no related to microbial activity.
I also feel that people tend to assume that water in S/H culture is stagnant. In my mind the constant flushing and replacement of water keeps the whole pot light and fresh. I tend to assume that there are far less anaerobic bacteria in S/H than most other styles of potting.
|
Isn't one of the main advantages to S/H avoiding work like this?
Quote:
|
Sorry if that was rant and I hope it made some sense. All that said stagnant water and bacteria also must kill roots of course but I have a sense they are not usually the cause of root death in repottings and situation changes
|
You made perfect sense, and I didn't detect a hint of rant.
Thanks for your post.
-Keith
---------- Post added at 01:33 PM ---------- Previous post was at 01:30 PM ----------
Quote:
Originally Posted by SouthPark

I haven't tried it yet. But wondering if an aquarium air-stone pump system for water surface air mixing can help with keeping regular roots alive - by keeping the water adequately oxygenated. But ..... my main reason for not going for water culture (not 'S.H.') is the need for fixing the position of the orchid in its water container. Maybe a bit fiddly if too many orchids.
|
Terracotta pots are an oxygen permeable container that can hold water. I put most of my larger orchids, such as cattleya, in slotted terracotta pots for this reason.
-Keith
---------- Post added at 01:45 PM ---------- Previous post was at 01:33 PM ----------
Quote:
Originally Posted by Ray

When I first was thinking about the “suffocation” of roots in media that was too fine - whether that be due to a case of poor medium choice or it becoming old and compressed, the term I used was “stifling gas exchange”, which is pretty nonspecific in regards to what gases and in what direction.
I suppose that over the years that has been shortened to “suffocation”.
|
As I noted in my earlier post, I can envision other mechanisms that can stifle gas exchange other than blocked pathways. One is the possibility that aerobic microbes consume the oxygen. Another is that microbes release toxins that stress the orchid roots. A third could be pH changes.
Let me ask your opinion on this. Do you think the often heard statements that the roots of some orchids such as C. walkeriana or Tolumnia will die if they don't dry quickly after watering?
-Keith
__________________
+++++++++++
|

01-11-2022, 05:18 PM
|
|
Senior Member
|
|
Join Date: Dec 2018
Location: Australia, North Queensland
Posts: 5,212
|
|
Quote:
Originally Posted by K-Sci

Terracotta pots are an oxygen permeable container that can hold water. I put most of my larger orchids, such as cattleya, in slotted terracotta pots for this reason.
|
That's a nice workable method. Over here, I have only used plastic opaque pots in the tropics with very good (and lots of) drainage holes down the bottom - works nicely too. I just do something along the lines shown in this pic here .... Pic Link.
Sometimes - I use much larger pots than usual, and only use smaller media for the surface region (depth of my own choice) with relatively small orchids (such as seedlings, or some juveniles) - otherwise, I just use one sort of media purchased with some average size.
Even though the image shows watering toward the rim or outskirts of the pot ----- that doesn't strictly need to be followed. I just dump most of the water at the outskirts, and have the choice to add water further in. |
|
Currently Active Users Viewing This Thread: 1 (0 members and 1 guests)
|
|
|
 Posting Rules
Posting Rules
|
You may not post new threads
You may not post replies
You may not post attachments
You may not edit your posts
HTML code is Off
|
|
|
All times are GMT -4. The time now is 11:06 PM.
|
































 Linear Mode
Linear Mode


